RNA: The Emergence of CircRNA Therapies
Author: Sophia Shamsi, Senior Market Analyst, Hanson Wade Intelligence
An emerging field
In recent years circRNA has emerged as an attractive target, therapeutic modality, and diagnostic agent. Focusing on circRNA as a therapeutic modality the drug class offers broad applicability and the potential to overcome the stability challenges faced by linear RNA. These properties have positioned circRNA as a promising next-generation RNA modality. The potential of the drug class coupled with increased interest and investment in the RNA space has triggered a wave of activity directed at the modality. To explore this activity, we have examined the drug data in Beacon.
In 2021, the first circRNA therapy entered development, since, a further 46 assets have been added to the pipeline. The pipeline growth between 2021-2022 was significant, with an annual growth rate of 154%. The graph below shows the number of circRNA assets added to the pipeline per year (fig.1).
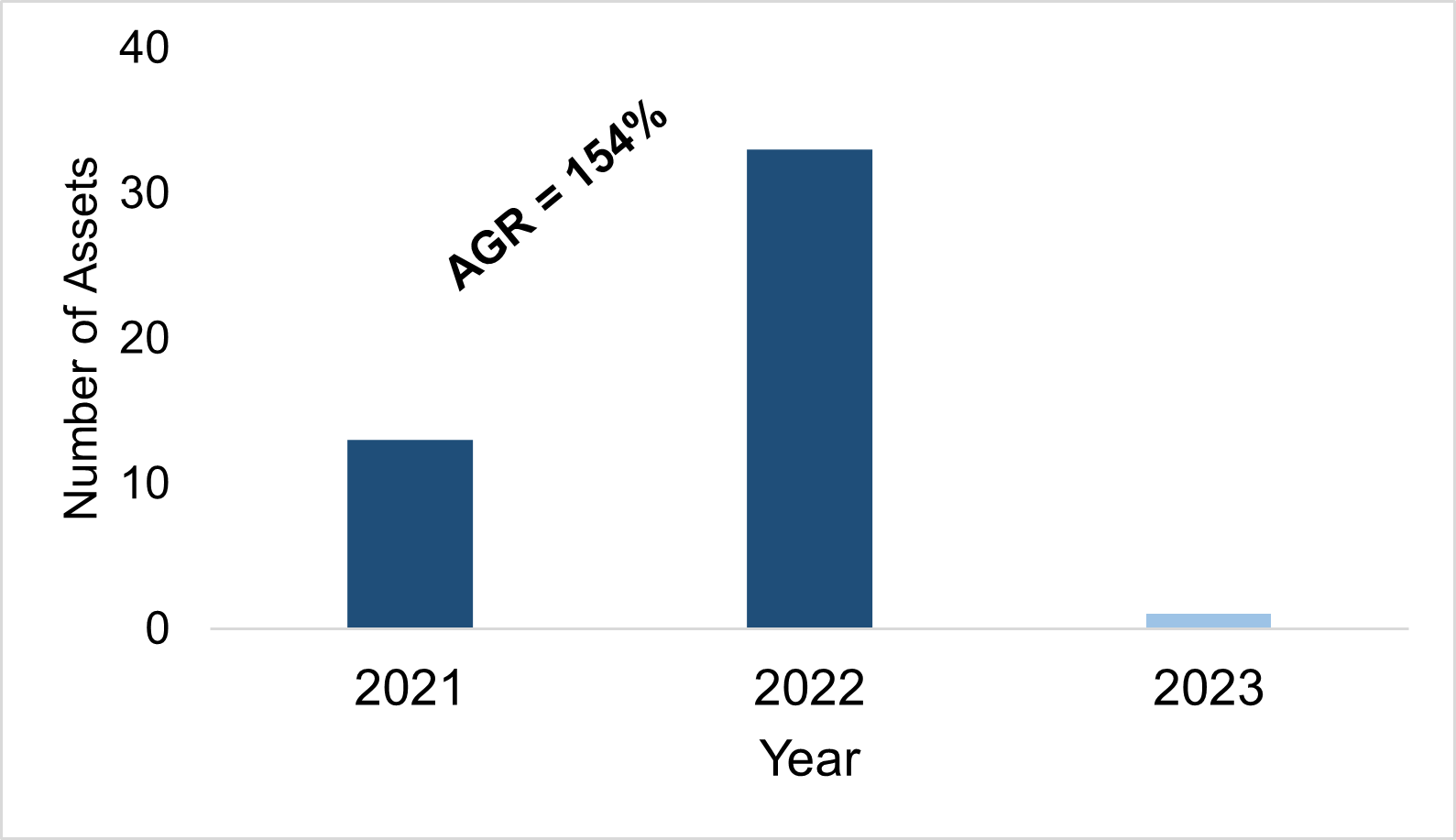
Figure. 1 The number of circRNA assets added to the pipeline per year.
Source: Beacon RNA
Although all assets remain in discovery and preclinical development, the developer landscape appears competitive and diverse. As of Feb 2023, 18 developers are operating within the space, these developers range from circRNA-focused biotechs to big pharma, such as Merck. As the innovation sentiment within the space strengthens, and developers seek to expand their RNA pipelines, we can expect to see an increase in investment and collaborations.
An innovative landscape
Although circRNA has presented promise, a range of challenges remain. Platform technologies, which have played a significant role in the success of RNA therapeutics to date, are being developed to address these challenges. Within the circRNA field a total of 8 disclosed platform technologies have been developed. An example of current innovations include Orna Therapeutics’ oRNATM-LNP and isCARTM technologies which, when combined, leverage the capabilities of both circRNA and in situ CAR to overcome the existing challenges faced by ex vivo CAR-T therapies. The ability to combine two promising modalities highlights the broad applicability of the drug class and is something we anticipate to be a strategy employed by other developers. Further, with use of these technologies, we can expect to see accelerated development of circRNA therapies.
Looking forward
The circRNA field is rapidly expanding and is yet to stabilize, based on this we may expect to see further innovations and pipeline growth in addition to a continuously fruitful financial landscape. With preclinical research underway the validity of the modality is soon to be established, subsequently, we may see an increase in activity as companies seek to enter the space in its youth.
—————————————————————–
References:
– Beacon RNA
– https://www.ornatx.com/our-approach-strategy/
For further reading, download our recent report ‘Biopharma Deals in RNA Space: A post-Covid Landscape Review‘ where you’ll gain valuable insights on the main industry movement in the RNA space and development of RNA therapies.